At what point will a buffer solution cease to moderate changes in pH?
A) when waters equilibrium constant 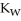 has been reached
has been reached
B) when the weak acid and the weak base salt concentration are equal
C) when the acting buffering component is all neutralized
D) only after all the acid or the base that needs to be buffered has been added
Correct Answer:
Verified
Q93: What happens to the pH of soda
Q104: When a hydronium ion concentration equals 1
Q105: In the following buffer system, what happens
Q106: In the following buffer system, what happens
Q106: In the following buffer system, what happens
Q107: Sodium hydroxide is added to a buffer
Q108: What is the hydroxide ion concentration in
Q112: A buffer solution can neutralize _.
A)strong acids
Q113: Hydrogen chloride is added to a buffer
Q114: Which of the following statements about buffers
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents