What is the hydroxide ion concentration in an aqueous solution when the hydronium ion concentration equals 1 × 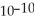 moles per liter?
moles per liter?
A) The concentration of hydroxide ions is 1 × 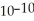 moles per liter.
moles per liter.
B) The concentration of hydroxide ions is 1 × 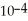 moles per liter.
moles per liter.
C) The concentration of hydroxide ions is 1 × 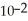 moles per liter.
moles per liter.
D) The concentration of hydroxide ions is 1 × 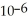 moles per liter.
moles per liter.
Correct Answer:
Verified
Q93: What happens to the pH of soda
Q103: When the pH of a solution is
Q104: When a hydronium ion concentration equals 1
Q105: In the following buffer system, what happens
Q106: In the following buffer system, what happens
Q106: In the following buffer system, what happens
Q107: Sodium hydroxide is added to a buffer
Q109: At what point will a buffer solution
Q112: A buffer solution can neutralize _.
A)strong acids
Q113: Hydrogen chloride is added to a buffer
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents