The following set of redox reactions takes place as shown. 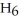 If you had two containers filled with the ion solution described above with only a wire connecting a piece of iron (in container A) and a piece of copper (in container B) , which way do the electrons flow?
If you had two containers filled with the ion solution described above with only a wire connecting a piece of iron (in container A) and a piece of copper (in container B) , which way do the electrons flow?
A) There is no continuous flow of electrons.
B) The electrons from from container A to container B.
C) The electrons flow from container B to container A.
D) The electrons flow back and forth between the two containers.
E) The electrons only move in the containers they originate in.
Correct Answer:
Verified
Q50: The following set of redox reactions takes
Q51: The following set of redox reactions takes
Q52: Glucose, Q55: The following set of redox reactions takes Q57: The zinc within a copper penny does Q58: Why is it easier for the body Q123: Which of the following statements best describes Q132: Which of the following statements about electrochemistry Q138: The following set of redox reactions takes Q139: Which of the following statements best describes![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents