The following set of redox reactions takes place as shown. 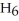 If you had two containers filled with the ion solution described above with a wire connecting a piece of iron (in container A) and a piece of copper (in container B) and a salt-bridge connecting the two containers, describe what happens.
If you had two containers filled with the ion solution described above with a wire connecting a piece of iron (in container A) and a piece of copper (in container B) and a salt-bridge connecting the two containers, describe what happens.
A) A little bit of iron is converted into iron ions and a little bit of copper ion is converted into copper metal.
B) All of the iron is transformed into iron ions and all of the copper is transformed into copper ions.
C) All of the iron is transformed into iron ions and all of the copper ions are transformed into copper metal.
D) All of the iron ions are transformed into iron metal and all of the copper is transformed into copper ions.
E) All of the iron ions are transformed into iron metal and all of the copper is transformed into copper metal.
Correct Answer:
Verified
Q45: In the oxidation-reduction reaction Mg(s)+ 
Q46: Upon ingestion, grain alcohol, Q51: The following set of redox reactions takes Q52: Glucose, Q53: The following set of redox reactions takes Q55: The following set of redox reactions takes Q132: Which of the following statements about electrochemistry Q134: What is the purpose of a salt-bridge? Q138: The following set of redox reactions takes Q139: Which of the following statements best describes![]()
![]()
A)to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents