What is the purpose of the salt bridge in a voltaic cell?
A) to prevent any further migration of electrons through the wire
B) to allow for the build up of positively charged ions in one container and negatively charged ions in the other container
C) to allow the 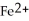 and the
and the 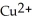 to flow freely between the two containers
to flow freely between the two containers
D) to allow for a balance of charge between the two chambers
Correct Answer:
Verified
Q55: The following set of redox reactions takes
Q57: The zinc within a copper penny does
Q58: Why is it easier for the body
Q59: The following set of redox reactions takes
Q62: Some car batteries require the periodic addition
Q64: Zinc, Zn, is more easily oxidized than
Q123: Which of the following statements best describes
Q124: In a battery,the following oxidation-reduction reactions are
Q145: Your car lights were left on while
Q159: In a battery,the following oxidation-reduction reactions are
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents