Zinc, Zn, is more easily oxidized than copper, Cu. What happens the moment after metal strips of these metals come into contact with each other?
A) The zinc metal immediately begins depositing electrons onto the surface of the copper, thus coating the copper with a white powder of zinc oxide, ZnO.
B) The zinc metal begins to turn a greenish color indicating the presence of the formation of cupric oxide, CuO.
C) The surface of the copper appears moist and cloudy as the zinc metal oxidizes and gets dissolved into 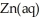 ions.
ions.
D) Nothing happens. An immediate build up of charge in either material prevents continued oxidation-reduction from occurring.
Correct Answer:
Verified
Q59: The following set of redox reactions takes
Q60: What is the purpose of the salt
Q62: Some car batteries require the periodic addition
Q67: Why is the anode of a battery
Q124: In a battery,the following oxidation-reduction reactions are
Q126: In a battery,the following two oxidation-reduction reactions
Q131: In a battery,the following two oxidation-reduction reactions
Q143: Why does a battery that has thick
Q145: Your car lights were left on while
Q159: In a battery,the following oxidation-reduction reactions are
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents