The molecular geometry of the left-most carbon atom in the molecule below is ________. 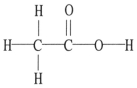
A) trigonal planar
B) trigonal bipyramidal
C) tetrahedral
D) octahedral
E) T-shaped
Correct Answer:
Verified
Q5: The molecular geometry of the BrO3- ion
Q10: The basis of the VSEPR model of
Q11: Of the following species,_ will have bond
Q14: The bond angles marked a, b, and
Q15: The electron-domain geometry of _ is tetrahedral.
A)CBr4
B)PH3
C)CCl2Br2
D)XeF4
E)all
Q16: Of the molecules below,only _ is polar.
A)CCl4
B)CH4
C)SeF4
D)SiCl4
Q18: PCl5 has _ electron domains and a
Q19: In counting the electron domains around the
Q22: The hybridization of the oxygen atom labeled
Q39: The hybridizations of bromine in BrF5 and
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents