The hybridization of the oxygen atom labeled y in the structure below is ________. The C-O-H bond angle is ________. 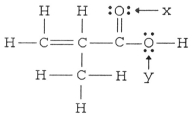
A) sp, 180°
B) sp2, 109.5°
C) sp3, 109.5°
D) sp3d2, 90°
E) sp, 90°
Correct Answer:
Verified
Q5: The molecular geometry of the BrO3- ion
Q15: The electron-domain geometry of _ is tetrahedral.
A)CBr4
B)PH3
C)CCl2Br2
D)XeF4
E)all
Q17: The molecular geometry of the left-most carbon
Q19: In counting the electron domains around the
Q22: For molecules with only one central atom,how
Q23: Of the following molecules, only _ is
Q24: The hybrid orbitals used for bonding by
Q24: The sp3d2 atomic hybrid orbital set accommodates
Q28: Of the following molecules,only _ is polar.
A)CCl4
B)BCl3
C)NCl3
D)BeCl2
E)Cl2
Q39: The hybridizations of bromine in BrF5 and
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents