The hybridization of the carbon atom labeled x in the molecule below is ________. 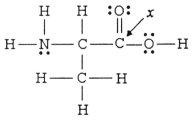
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2
Correct Answer:
Verified
Q65: Electrons in _ bonds remain localized between
Q68: The bond order of any molecule containing
Q78: Based on molecular orbital theory, there is/are
Q79: Structural changes around a double bond in
Q79: In comparing the same two atoms bonded
Q83: Of the following, only _ appears to
Q86: The molecular geometry of _ is square
Q90: The molecular geometry of the CHCl3 molecule
Q94: The molecular geometry of the PF4+ ion
Q95: According to MO theory,overlap of two p
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents