Of the acids in the table below, ________ is the strongest acid. 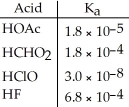
A) HOAc
B) HCHO2
C) HClO
D) HF
E) HOAc and HCHO2
Correct Answer:
Verified
Q2: The molar concentration of hydronium ion in
Q3: Which one of the following statements regarding
Q9: The magnitude of Kw indicates that _.
A)water
Q12: Which one of the following is the
Q13: A Br∅nsted-Lowry base is defined as a
Q14: Classify the following compounds as weak acids
Q16: The hydride ion, H-, is a stronger
Q17: Of the following acids,_ is not a
Q21: Using the data in the table, which
Q22: Using the data in the table, which
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents