Using the data in the table, which of the conjugate acids below is the weakest acid? 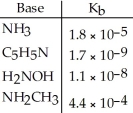
A) NH4+
B) C5H5NH+
C) H3NOH+
D) NH3CH3+
E) NH4+ and NH3CH3+
Correct Answer:
Verified
Q2: The molar concentration of hydronium ion in
Q13: A Br∅nsted-Lowry base is defined as a
Q16: The hydride ion, H-, is a stronger
Q17: Of the following acids,_ is not a
Q17: Of the acids in the table below,
Q22: Using the data in the table, which
Q25: Which of the following aqueous solutions has
Q26: A- is a weak base. Which equilibrium
Q35: Ammonia is a _.
A)weak acid
B)strong base
C)weak base
D)strong
Q37: Which of the following ions will act
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents