The formal charge on nitrogen in NO3- is __________. 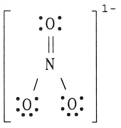
A) -1
B) 0
C) +1
D) +2
E) -2
Correct Answer:
Verified
Q29: Bond enthalpy is _.
A)always positive
B)always negative
C)sometimes positive,
Q71: Electronegativity _ from left to right within
Q74: Electropositivity _ from left to right within
Q78: The ion NO- has _ valence electrons.
A)15
B)14
C)16
D)10
E)12
Q85: In the Lewis structure of HCO3-,the formal
Q87: In the Lewis structure of ClF,the formal
Q87: How many equivalent resonance forms can be
Q88: The formal charge on sulfur in SO42-
Q91: The Lewis structure of PF3 shows that
Q94: The ion ICI4- has _ valence electrons.
A)34
B)35
C)36
D)28
E)8
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents