The formal charge on sulfur in SO42- is __________, where the Lewis structure of the ion is: 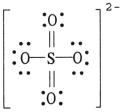
A) -2
B) 0
C) +2
D) +4
E) -4
Correct Answer:
Verified
Q29: Bond enthalpy is _.
A)always positive
B)always negative
C)sometimes positive,
Q73: The ion PO43- has _ valence electrons.
A)14
B)24
C)27
D)29
E)32
Q78: The ion NO- has _ valence electrons.
A)15
B)14
C)16
D)10
E)12
Q83: The ability of an atom in a
Q85: The formal charge on carbon in the
Q85: In the Lewis structure of HCO3-,the formal
Q86: How many equivalent resonance forms can be
Q87: How many equivalent resonance forms can be
Q87: In the Lewis structure of ClF,the formal
Q92: The formal charge on nitrogen in NO3-
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents