A sample of a gas (1.50 mol) is contained in a 15.0 L cylinder. The temperature is increased from 100°C to 150°C. The ratio of final pressure to initial pressure 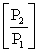 is __________.
is __________.
A) 1.50
B) 0.667
C) 0.882
D) 1.13
E) 1.00
Correct Answer:
Verified
Q42: A sample of H2 gas (12.28 g)occupies
Q44: A sample of a gas originally at
Q46: A gas at a pressure of 325
Q50: A gas at a pressure of 10.0
Q54: A sample of a gas originally at
Q61: The reaction of 50 mL of Cl2
Q63: The reaction of 50 mL of Cl2
Q67: The reaction of 25 mL of N2
Q71: A gas vessel is attached to an
Q75: The amount of gas that occupies 60.82
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents