The Keq for the equilibrium below is 7.73 × 10-2 at 500.0°C. 2Cl2 (g) + 2H2O (g) 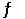 4HCl (g) + O2 (g)
4HCl (g) + O2 (g)
What is the value of Keq at this temperature for the following reaction?
Cl2 (g) + H2O (g)  2HCl (g) +
2HCl (g) + 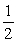 O2 (g)
O2 (g)
A) 0.0773
B) 5.98 × 10-3
C) 0.278
D) 0.0376
E) 0.150
Correct Answer:
Verified
Q72: Phosphorous trichloride and phosphorous pentachloride equilibrate in
Q73: At 200°C, the equilibrium constant (Kp)for the
Q74: At 900.0 K, the equilibrium constant (Kp)for
Q75: Given the following reaction at equilibrium, if
Q76: The Kp for the reaction below is
Q79: Dinitrogen tetroxide partially decomposes according to the
Q83: If the value for the equilibrium constant
Q87: If Reaction A + Reaction B =
Q93: If the reaction quotient Q for a
Q96: If a reaction is endothermic, _ the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents