The Kp for the reaction below is 1.49 × 108 at 100.0°C: CO (g) + Cl2 (g) 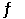 COCl2 (g)
COCl2 (g)
In an equilibrium mixture of the three gases, PCO = PCl2 = 2.22 × 10-4 atm. The partial pressure of the product, phosgene (COCl2) , is __________ atm.
A) 7.34
B) 3.02 × 1015
C) 3.31 × 10-16
D) 3.31 × 104
E) 6.67 × 1011
Correct Answer:
Verified
Q71: In the coal-gasification process, carbon monoxide is
Q72: Phosphorous trichloride and phosphorous pentachloride equilibrate in
Q73: At 200°C, the equilibrium constant (Kp)for the
Q74: At 900.0 K, the equilibrium constant (Kp)for
Q75: Given the following reaction at equilibrium, if
Q77: The Keq for the equilibrium below is
Q79: Dinitrogen tetroxide partially decomposes according to the
Q83: If the value for the equilibrium constant
Q87: If Reaction A + Reaction B =
Q93: If the reaction quotient Q for a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents