The equilibrium position corresponds to which letter on the graph of G vs. f (course of reaction) below? 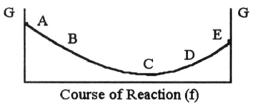
A) A
B) B
C) C
D) D
E) E
Correct Answer:
Verified
Q17: ΔS is positive for the reaction _.
A)2
Q25: For an isothermal process,the entropy change of
Q26: ΔS is positive for the reaction _.
A)Pb(NO3)2
Q28: A reaction that is not spontaneous at
Q28: Which reaction produces a decrease in the
Q29: Which reaction produces a decrease in the
Q29: Which reaction produces an increase in the
Q30: Given the following table of thermodynamic data,
Q33: Of the following, the entropy of gaseous
Q39: Consider the reaction: Ag+ (aq)+ Cl- (aq)→
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents