Consider the reaction: Ag+ (aq) + Cl- (aq) → AgCl (s)
Given the following table of thermodynamic data, 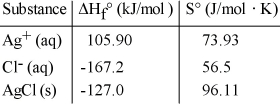 determine the temperature (in °C) above which the reaction is nonspontaneous under standard conditions.
determine the temperature (in °C) above which the reaction is nonspontaneous under standard conditions.
A) 1230
B) 150
C) 432
D) 133
E) 1640
Correct Answer:
Verified
Q25: For an isothermal process,the entropy change of
Q26: ΔS is positive for the reaction _.
A)Pb(NO3)2
Q28: Which reaction produces a decrease in the
Q29: Which reaction produces an increase in the
Q34: The equilibrium position corresponds to which letter
Q34: The value of ΔS° for the catalytic
Q37: The value of ΔS° for the reaction
Q40: Of the following, the entropy of _
Q50: The combustion of ethene in the presence
Q56: The combustion of hydrogen in the presence
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents