Consider the reaction coordinate diagram shown. What is the rate-determining step in the conversion of A to G? 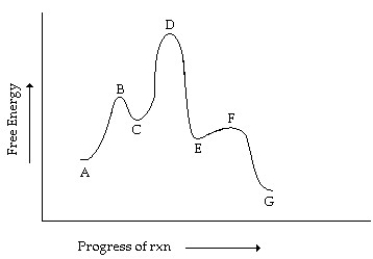
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q63: Under what conditions is ΔG° equal to
Q67: Based on the following energy diagram, which
Q68: Consider the reaction coordinate diagram shown. Which
Q69: Consider the single step interconversion of A
Q71: The ΔG° for the conversion of "axial"
Q71: Given an activation energy of 15 kcal/mol,
Q74: Consider the one-step conversion of F to
Q77: The Arrhenius equation models how the rate
Q78: Consider the conversion of C to D
Q80: What is the free energy of activation
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents