Box (1) represents 1.0 mL of a solution of particles at a given concentration. 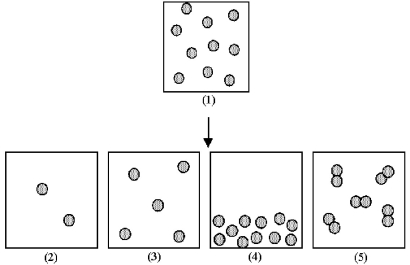
-Which of the boxes (2) -(5) represents 1.0 mL of the solution that results after (1) has been diluted by adding enough solvent to make 5.0 mL of solution?
A) box (2)
B) box (3)
C) box (4)
D) box (5)
Correct Answer:
Verified
Q108: What is the concentration of HCl in
Q109: Ascorbic acid,C6H806,can be represented by the molecular
Q110: How many milliliters of a 9.0 M
Q111: Assume that the unshaded spheres in the
Q112: A hydrocarbon of unknown formula CxHy was
Q114: A hydrocarbon of unknown formula CxHy was
Q115: Based on the positions in the periodic
Q116: Box (1)represents 1.0 mL of a solution
Q117: What volume of a 0.540 M NaOH
Q118: Based on the positions in the periodic
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents