Assume that the unshaded spheres in the buret represent H+ ions,the shaded spheres in the flask represent OH- ions,and you are carrying out a titration of the base with the acid. 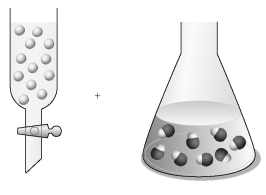
-If the volumes in the buret and the flask are identical and the concentration of the acid in the buret is 0.500 M,what is the concentration of the base in the flask?
A) 0.333 M
B) 0.500 M
C) 0.667 M
D) 0.750 M
Correct Answer:
Verified
Q106: Glucose,C6H1206,can be represented by the molecular model
Q107: What is the concentration of 200.mL of
Q108: What is the concentration of HCl in
Q109: Ascorbic acid,C6H806,can be represented by the molecular
Q110: How many milliliters of a 9.0 M
Q112: A hydrocarbon of unknown formula CxHy was
Q113: Box (1)represents 1.0 mL of a solution
Q114: A hydrocarbon of unknown formula CxHy was
Q115: Based on the positions in the periodic
Q116: Box (1)represents 1.0 mL of a solution
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents