Multiple Choice
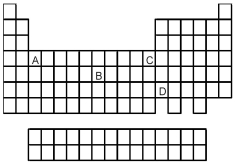
-Which element,indicated by letter on the periodic table above,has a 1+ ion with the electron configuration 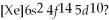
A) A
B) B
C) C
D) D
Correct Answer:
Verified
Related Questions
Q48: Calculate the lattice energy for MgO(s)using a
Q49: Calculate the energy change in kJ/mol for
Q50: The four spheres below represent Na+,Mg2+,F⁻,and O2-,not
Q51: The following four spheres represent a metal
Q52: The following four spheres represent a Na
Q54: Q55: Calculate the energy change for the formation Q56: The following four spheres represent a metal Q57: Q58: Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
![]()
![]()

