The following four spheres represent a Na atom,a Na+ ion,a Cl atom,and a Cl- ion,not necessarily in that order.Use your knowledge about the relative sizes of atoms,cations,and anions to determine which of the following sets of reactions is most consistent with the sizes of the atoms and ions shown below. 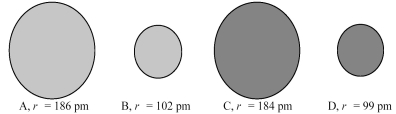
A) A → B + e- and C → D + e-
B) A → B + e- and D → C + e-
C) B → A + e- and C → D + e-
D) B → A + e- and D → C + e-
Correct Answer:
Verified
Q47: The following four spheres represent a metal
Q48: Calculate the lattice energy for MgO(s)using a
Q49: Calculate the energy change in kJ/mol for
Q50: The four spheres below represent Na+,Mg2+,F⁻,and O2-,not
Q51: The following four spheres represent a metal
Q53: Q54: Q55: Calculate the energy change for the formation Q56: The following four spheres represent a metal Q57: Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
![]()
![]()

