Calculate the total quantity of heat required to convert 50.0 g of liquid CCl4(l) from 25.0°C to gaseous CCl4 at 76.8°C (the normal boiling point for CCl4) ? The specific heat of CCl4(l) is 0.857 J/(g ∙ °C) ,its heat of fusion is 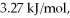 and its heat of vaporization is
and its heat of vaporization is 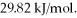
A) 2.22 kJ
B) 3.28 kJ
C) 11.91 kJ
D) 12.98 kJ
Correct Answer:
Verified
Q26: The enthalpy of fusion,or heat of fusion
Q27: Sodium metal reacts with water to produce
Q28: Water has an unusually high
A)electrical conductivity.
B)heat of
Q29: In the lab,you mix two solutions (each
Q30: When 50.0 mL of 0.400 M Ca(NO3)2
Q32: At 1 atm pressure the heat of
Q33: When 8.00 g of Ba(s)is added to
Q34: It takes 15.5 kJ of energy to
Q35: How much heat is absorbed/released when 10.00
Q36: How much heat is absorbed when 20.00
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents