When 5.50 g of Ba(s) is added to 100.00 g of water in a container open to the atmosphere,the reaction shown below occurs and the temperature of the resulting solution rises from 22.00°C to 61.16°C.If the specific heat of the solution is 4.18 J/(g ∙ °C) ,calculate 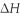 for the reaction,as written. Ba(s) + 2 H2O(l) → Ba(OH) 2(aq) + H2(g)
for the reaction,as written. Ba(s) + 2 H2O(l) → Ba(OH) 2(aq) + H2(g) 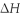 = ?
= ?
A) -431 kJ
B) -3.14 kJ
C) +3.14 kJ
D) +431 kJ
Correct Answer:
Verified
Q127: When 1.820 g of anthracene,C14H10,is combusted in
Q128: The specific heat of copper is 0.385
Q129: For which should the standard heat of
Q130: Find ΔH for BaCO3 (s)→ BaO (s)+
Q131: For the reaction,N H3(g)→ N(g)+ 3 H(g),one
Q133: Acetylene torches utilize the following reaction: 2
Q134: For which reaction is H expected to
Q135: How much heat is transferred per 5.00
Q136: Which is expected to have the largest
Q137: At a given temperature and pressure,which of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents