Multiple Choice
A phase diagram of temperature versus composition for a mixture of the two volatile liquids octane (bp = 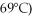 and decane (bp = 126°C) is shown.
and decane (bp = 126°C) is shown. 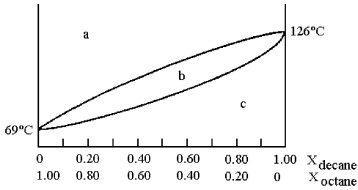
-Assume that you start with a mixture containing 0.80 mol of decane and 0.20 mol of octane,what region of the diagram corresponds to vapor?
A) region a
B) region b
C) region c
D) regions a and c
Correct Answer:
Verified
Related Questions
Q105: A phase diagram of temperature versus composition
Q106: A phase diagram of temperature versus composition

