Molecular hydrogen can be made from methane gas by the reaction below.How is the rate of disappearance of CH4 related to the rate of appearance of H2? - 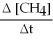 = ?
= ?
CH4 (g) + H2O (l) → CO (g) + 3H2 (g)
A) + 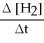
B) + 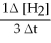
C) + 3 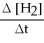
D) none of these
Correct Answer:
Verified
Q14: The reaction that occurs in a Breathalyzer,a
Q15: The decomposition of dinitrogen pentoxide is described
Q16: A concentration-time study of the gas phase
Q17: The following set of data was obtained
Q18: The hydrolysis of tert-butyl chloride is given
Q20: The decomposition of ammonia to nitrogen and
Q21: The isomerization reaction,CH3NC → CH3CN,is first order
Q22: The first-order reaction,SO2Cl2 → SO2 + Cl2,has
Q23: The decomposition of cyclopropane,was observed at 500°C
Q24: The data below were collected for the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents