The following set of data was obtained by the method of initial rates for the reaction: (H3C) 3CBr + OH- → (H3C) 3COH + Br-
What is the order of reaction with respect to ion,OH-? 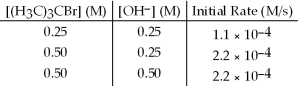
A) zero
B) first
C) second
D) none of these
Correct Answer:
Verified
Q32: The first-order reaction,SO2Cl2 → SO2 + Cl2,has
Q33: A zeroth order reaction is one whose
A)rate
Q34: Which statement below regarding the half-life of
Q35: The first-order reaction,2 N2O(g)→ 2 N2(g)+ O2(g),has
Q36: The following set of data was obtained
Q38: The following set of data was obtained
Q39: The following set of data was obtained
Q40: For a particular first-order reaction,it takes 48
Q41: In aqueous solution,hypobromite ion,BrO-,reacts to produce bromate
Q42: The reaction: 2 HI → H2 +
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents