The following set of data was obtained by the method of initial rates for the reaction: S2O82-(aq) + 3 I-(aq) → 2 SO42-(aq) + I3-(aq)
What is the initial rate when S2O82- is 0.15 M and I- is 0.15 M? 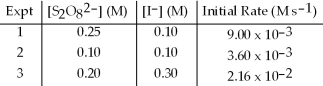
A) 4.10 × 10-6 M s-1
B) 8.10 × 10-3 M s-1
C) 1.22 × 10-2 M s-1
D) 5.40 × 10-2 M s-1
Correct Answer:
Verified
Q25: The half life of the reaction shown
Q27: The first-order decomposition of hydrogen peroxide occurs
Q28: The following reaction is first order: C2H6
Q29: The following set of data was obtained
Q31: Acetaldehyde decomposes at 750 K: CH3CHO →
Q32: The first-order reaction,SO2Cl2 → SO2 + Cl2,has
Q33: A zeroth order reaction is one whose
A)rate
Q34: Which statement below regarding the half-life of
Q35: The first-order reaction,2 N2O(g)→ 2 N2(g)+ O2(g),has
Q116: The rate constant,k,for a first-order reaction is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents