The reaction for the decomposition of dinitrogen monoxide gas to form an oxygen radical is: 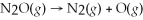 .If the activation energy is 250 kJ/mol and the frequency factor is 8.0 × 1011 s-1,what is the rate constant for the first-order reaction at 1000 K?
.If the activation energy is 250 kJ/mol and the frequency factor is 8.0 × 1011 s-1,what is the rate constant for the first-order reaction at 1000 K?
A) 1.1 × 10-3 s-1
B) 7.0 × 10-2 s-1
C) 1.6 × 1013 s-1
D) 9.1 × 1024 s-1
Correct Answer:
Verified
Q46: What is the minimum energy barrier that
Q47: The reaction for the decomposition of dinitrogen
Q48: A plot of 1/[BrO-] vs time is
Q49: For the hypothetical second order reaction: A
Q50: The second-order reaction,2 Mn(CO)5 → Mn2(CO)10 has
Q52: According to kinetic molecular theory,which of the
Q53: Nitrogen dioxide decomposes at 300°C via a
Q54: Which statement below regarding the half-life of
Q55: A gas molecule at 298 K and
Q56: When the temperature of a gas whose
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents