The relative initial rates of the reaction A2 + B2 → products in vessels (a) -(d) are 1:1:4:4.Unshaded spheres represent A2 molecules,and shaded spheres represent B2 molecules present at the beginning of the reaction. 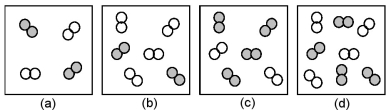
-What is the order of reaction with respect to A2?
A) 0
B) 1
C) 2
D) 3
Correct Answer:
Verified
Q92: The following reaction is second order in
Q93: Consider the first-order decomposition of A molecules
Q94: Consider the first-order decomposition of A molecules
Q95: Consider the first-order decomposition of A molecules
Q96: The following reaction is first order in
Q98: The following reaction is first order in
Q99: The relative initial rates of the reaction
Q100: The following reaction is second order in
Q101: Consider a reaction that occurs by the
Q102: ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents