Consider the first-order decomposition of A molecules (shaded spheres) in two vessels of equal volume.How will the rate of decomposition in vessel (a) be affected if the volume of the vessel is decreased by a factor of 2? 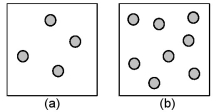
A) decrease by 1/2
B) increase by 2
C) increase by 4
D) stay the same
Correct Answer:
Verified
Q89: The following reaction is first order in
Q90: The relative initial rates of the reaction
Q91: Consider the first-order decomposition of A molecules
Q92: The following reaction is second order in
Q93: Consider the first-order decomposition of A molecules
Q95: Consider the first-order decomposition of A molecules
Q96: The following reaction is first order in
Q97: The relative initial rates of the reaction
Q98: The following reaction is first order in
Q99: The relative initial rates of the reaction
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents