Find the equilibrium constant for the reaction: A(g) + B(g) ⇌ 2C(g) at 25°C when k equals 1.4 × 10-12 M-1s-1 for the reaction A(g) + B(g) → 2C(g) at 25°C and k equals 2.7 × 10-13 M-1s-1 for the reaction: 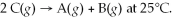
A) 3.8 × 10-25
B) 1.7 × 10-12
C) 1.1 × 10-12
D) 5.2
Correct Answer:
Verified
Q57: The esterification of acetic acid and ethanol
Q58: The following two isomers of C3H7NO exist
Q59: Acids donate protons to water according to
Q60: For the isomerization reaction: butane ⇌ isobutane
Kp
Q61: The pink and blue species below form
Q63: Consider the interconversion of A molecules (shaded
Q64: A catalyst increases the rate of a
Q65: What effect will a change in temperature
Q66: The following pictures represent mixtures of cis-C2H2X2
Q67: The dissolution of calcium hydroxide is exothermic:
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents