Consider the interconversion of A molecules (shaded spheres) and B molecules (unshaded spheres) according to the reaction A ⇌ B.Each of the following series of pictures represents a separate experiment in which time increases from left to right. 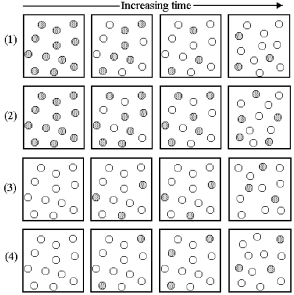
-What is the value of the equilibrium constant Kc for the reaction A ⇌ B?
A) Kc = 0.33
B) Kc = 3.0
C) Kc = 12
D) Kc = 27
Correct Answer:
Verified
Q58: The following two isomers of C3H7NO exist
Q59: Acids donate protons to water according to
Q60: For the isomerization reaction: butane ⇌ isobutane
Kp
Q61: The pink and blue species below form
Q62: Find the equilibrium constant for the reaction:
Q64: A catalyst increases the rate of a
Q65: What effect will a change in temperature
Q66: The following pictures represent mixtures of cis-C2H2X2
Q67: The dissolution of calcium hydroxide is exothermic:
Q68: Calcium carbonate is relatively insoluble and the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents