The following pictures represent the equilibrium state for four different reactions of the type 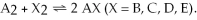 A atoms are unshaded.X atoms are shaded.
A atoms are unshaded.X atoms are shaded. 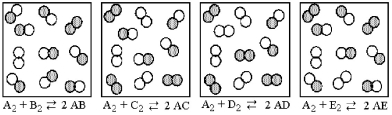
-Which reaction has the largest equilibrium constant?
A) A2 + B2 ⇌ 2 AB
B) A2 + C2 ⇌ 2 AC
C) A2 + D2 ⇌ 2 AD
D) A2 + E2 ⇌ 2 AE
Correct Answer:
Verified
Q70: Which of the following will result in
Q71: The hexaammine cobalt(III)ion is very unstable in
Q72: Consider the interconversion of A molecules (shaded
Q73: A reaction reaches dynamic equilibrium at a
Q74: The reaction A2 + B2 ⇌ 2
Q76: Nickel metal can be prepared by the
Q77: The reaction below virtually goes to completion
Q78: A catalyst increases the overall rate of
Q79: At 25°C,a certain first order reaction has
Q80: The following pictures represent the equilibrium state
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents