The following pictures represent solutions that contain a weak acid HA (pKa = 5.0) and its potassium salt KA.Unshaded spheres represent H atoms and shaded spheres represent A- ions.(K+,H3O+,OH-,and solvent H2O molecules have been omitted for clarity. ) 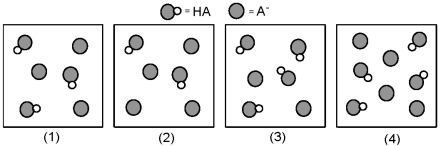
-For which of these solutions is pH = pKa?
A) All have pH = pKa.
B) (1) , (2) and (3)
C) (1) and (4)
D) (2) and (3)
Correct Answer:
Verified
Q62: The following pictures represent solutions that contain
Q68: The following pictures represent solutions that contain
Q68: The following pictures represent solutions that contain
Q70: The following pictures represent solutions that contain
Q70: The following pictures represent solutions that contain
Q74: Precipitation of an ionic compound will occur
Q75: Potassium chromate is slowly added to a
Q76: The following pictures represent solutions that contain
Q77: The following pictures represent solutions that contain
Q78: A solution may contain the following ions
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents