The figure represents the spontaneous evaporation of nitrogen in which liquid nitrogen,N2(l) ,becomes gaseous nitrogen,N2(g) : N2(l) → N2(g) .What are the signs (+ or -) of ΔH,ΔS,and ΔG for this process? 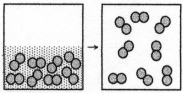
A) ΔH = +,ΔS = +,ΔG = +
B) ΔH = +,ΔS = +,ΔG = -
C) ΔH = -,ΔS = -,ΔG = +
D) ΔH = -,ΔS = -,ΔG = -
Correct Answer:
Verified
Q70: Q71: At high temperatures,boron carbide vaporizes according to Q72: Solid NaHCO3 is heated to 90°C.At equilibrium Q73: The figure represents the spontaneous deposition of Q74: If ΔG° is positive for a reaction, Q76: What is the relationship between ΔG,Qp,and Kp Q77: In figure (1)below argon atoms,represented by unshaded![]()
A)K
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents