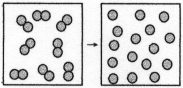
-The figure above represents the nonspontaneous reaction O2(g) → 2O(g) .What are the signs (+ or -) of ΔH,ΔS,and ΔG for this process?
A) ΔH = +,ΔS = +,ΔG = +
B) ΔH = +,ΔS = +,ΔG = -
C) ΔH = -,ΔS = -,ΔG = +
D) ΔH = -,ΔS = -,ΔG = -
Correct Answer:
Verified
Q65: If ΔG is small and positive,
A)the forward
Q66: Calculate Ksp for PbI2 at 25°C based
Q67: For the following reaction find Kp at
Q68: If Q increases,
A)ΔG increases and the reaction
Q69: Which of the following must be true
Q71: At high temperatures,boron carbide vaporizes according to
Q72: Solid NaHCO3 is heated to 90°C.At equilibrium
Q73: The figure represents the spontaneous deposition of
Q74: If ΔG° is positive for a reaction,
A)K
Q75: The figure represents the spontaneous evaporation of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents