Consider the reaction 2A(g) ⇌ A2(g) .The following pictures represent two possible initial states and the equilibrium state of the system. 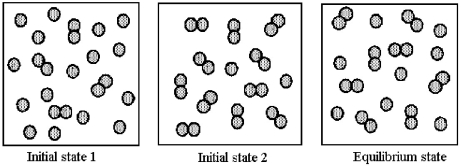
-What are the signs (+ or -) of ΔH,ΔS,and ΔG when the system spontaneously goes from initial state 2 to the equilibrium state?
A) ΔH = +,ΔS = +,ΔG = +
B) ΔH = +,ΔS = +,ΔG = -
C) ΔH = -,ΔS = -,ΔG = +
D) ΔH = -,ΔS = -,ΔG = -
Correct Answer:
Verified
Q83: Consider the following gas-phase reaction of A2
Q84: Which of the following processes is spontaneous?
A)a
Q85: What is the entropy change associated with
Q86: For which of the following will the
Q87: The figure below represents the spontaneous reaction
Q89: Q90: For which process is the sign of Q91: Consider the reaction 2A(g)⇌ A2(g).The following pictures Q92: The figure below represents the spontaneous reaction Q93: ![]()
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents