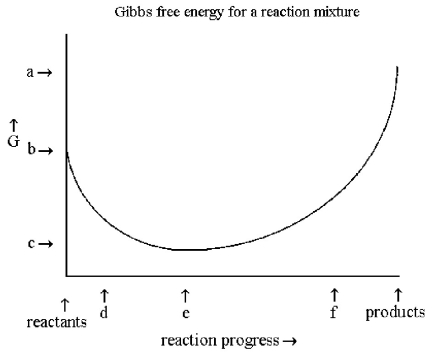
-According to the diagram above,
A) ΔG° is positive and is equal to a - b.
B) ΔG° is positive and is equal to b - c.
C) ΔG° is negative and is equal to b - c.
D) ΔG° is negative and is equal to a - c.
Correct Answer:
Verified
Q84: Which of the following processes is spontaneous?
A)a
Q85: What is the entropy change associated with
Q86: For which of the following will the
Q87: The figure below represents the spontaneous reaction
Q88: Consider the reaction 2A(g)⇌ A2(g).The following pictures
Q90: For which process is the sign of
Q91: Consider the reaction 2A(g)⇌ A2(g).The following pictures
Q92: The figure below represents the spontaneous reaction
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents