Consider the galvanic cell shown below. 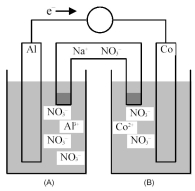
-Identify the anode and cathode,and indicate the direction of ion flow to and from each electrode.
A) Al is the anode and Co is the cathode;Al3+ ions flow to the anode and Co2+ ions flow from the cathode.
B) Al is the anode and Co is the cathode;Co2+ ions flow to the cathode and Al3+ ions flow from the anode.
C) Co is the anode and Al is the cathode;Al3+ ions flow to the cathode and Co2+ ions flow from the anode.
D) Co is the anode and Al is the cathode;Co2+ ions flow to the anode and Al3+ ions flow from the cathode.
Correct Answer:
Verified
Q104: Consider the galvanic cell shown below.
Q105: Consider the following galvanic cell. 
Q106: Consider the galvanic cell shown below.
Q107: Consider the following galvanic cell. 
Q108: Consider the following galvanic cell. 
Q110: Consider the following galvanic cell. 
Q111: NaNO3(aq)is employed in the salt bridge.Give the
Q112: If the concentrations of Ag+(aq)and Cu2+(aq)are varied
Q113: Given the half-cell potentials below,calculate the cell
Q114: Identify the anode and cathode half-reactions and
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents