Consider the following galvanic cell. 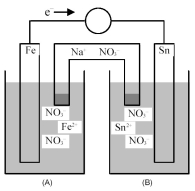
-What is the balanced equation for the cell reaction?
A) Fe(s) + Sn(s) → Fe2+(aq) + Sn2+(aq)
B) Fe2+(aq) + Sn2+(aq) → Fe(s) + Sn(s)
C) Fe(s) + Sn2+(aq) → Fe2+(aq) + Sn(s)
D) Fe2+(aq) + Sn(s) → Fe(s) + Sn2+(aq)
Correct Answer:
Verified
Q100: The chlor-alkali industry is based on the
Q101: Shown below is a galvanic cell with
Q102: Q103: Shown below is a galvanic cell with Q104: Consider the galvanic cell shown below. Q106: Consider the galvanic cell shown below. Q107: Consider the following galvanic cell. Q108: Consider the following galvanic cell. Q109: Consider the galvanic cell shown below. Q110: Consider the following galvanic cell. ![]()



Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents