Shown below is an electrochemical cell with anode a and cathode c.Both the anode and the cathode are inert electrodes.The liquid shown in compartment b of the cell is molten potassium chloride,KCl(l) . 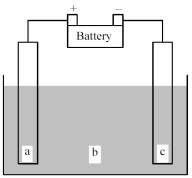
-Determine whether this is a galvanic or an electrolytic cell and give the reaction occurring at the anode and the reaction occurring at the cathode.
A) Electrolytic cell
Anode reaction: K(s) → K+(l) + e-
Cathode reaction: Cl2(g) + 2e- → 2 Cl-(l)
B) Electrolytic cell
Anode reaction: 2 Cl-(l) → Cl2(g) + 2e-
Cathode reaction: K+(l) + e- → K(s)
C) ..Galvanic cell
Anode reaction: K(s) → K+(l) + e-
Cathode reaction: Cl2(g) + 2e- → 2 Cl-(l)
D) .Galvanic cell
Anode reaction: K(s) → K+(l) + e-
Cathode reaction: Cl2(g) + 2e- → 2 Cl-(l)
Correct Answer:
Verified
Q120: Q121: Based on the balanced chemical equation shown Q122: According to the balanced equation shown below,3.00 Q123: The initial concentrations of Ag+(aq)and Cu2+(aq)are both Q124: Consider the following galvanic cell. Q126: Based on the balanced chemical equation shown Q127: What are the coefficients in front of![]()

Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents