Shown below is an electrochemical cell with anode a and cathode c.Both the anode and the cathode are inert electrodes.The liquid shown in compartment b of the cell is molten potassium chloride,KCl(l) . 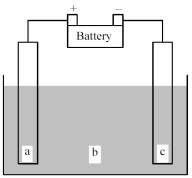
-Determine the direction of electron flow and the direction of ion flow.
A) Electrons flow from a to c;K+ ions flow toward a and Cl- ions flow toward c.
B) Electrons flow from a to c;Cl- ions flow toward a and K+ ions flow toward c.
C) Electrons flow from c to a;K+ ions flow toward a and Cl- ions flow toward c.
D) Electrons flow from c to a;Cl- ions flow toward a and K+ ions flow toward c.
Correct Answer:
Verified
Q125: Shown below is an electrochemical cell with
Q126: Based on the balanced chemical equation shown
Q127: What are the coefficients in front of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents