What is the relationship between the standard cell potentials,E°,for the following two galvanic cell reactions? I.3 Cu2+(aq) + 2 Al(s) → 3 Cu(s) + 2 Al3+(aq)
II.30 Cu2+(aq) + 22 Al(s) → 30 Cu(s) + 22 Al3+(aq)
A) E°(I) = E°(II)
B) E°(I) = 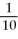
E°(II)
C) E°(I) = 10E°(II)
D) E°(I) = E°(II) 10
Correct Answer:
Verified
Q171: Using the following standard reduction potentials, Fe3+(aq)+
Q172: What is the standard cell potential for
Q173: What is the value of the equilibrium
Q174: What is the relationship between the standard
Q175: Calculate the equilibrium constant,K,at 25°C for the
Q177: Calculate the value of the reaction quotient,Q,for
Q178: Which battery does not use MnO2(s)as a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents