In the reaction shown,what is the mole ratio that would be used to determine the number of moles of H2 that would be produced when 3.5 moles of AlCI3 are produced?
2 Al + 6 HCl → 2 AlCI3 + 3 H2
A) 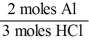
B) 
C) 
D) 
E) 
Correct Answer:
Verified
Q21: The number of grams in 2.65 mol
Q22: In the balanced reaction shown,the mole ratio
Q26: Interpret in words the equation:
P4O10 (s)+ 6
Q28: Consider the reaction N2 (g)+ O2 (g)→
Q32: In the reaction shown,what is the mole
Q35: In the reaction shown,how many moles of
Q40: The number of grams in 7.00 moles
Q43: How many grams of C will be
Q47: The percentage yield of a reaction can
Q49: How many grams of fluorine are required
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents