In the reaction shown,what is the mole ratio that would be used to determine the number of moles of oxygen needed to react with 3.2 moles of C4H10?
2 C4H10 + 13 O2 → 8 CO2 + 10 H2O
A) 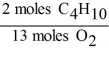
B) 
C) 
D) 
E) 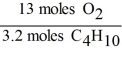
Correct Answer:
Verified
Q21: The number of grams in 2.65 mol
Q27: In the reaction shown,what is the mole
Q27: How many moles of NaHCO3 are present
Q28: Consider the reaction N2 (g)+ O2 (g)→
Q33: How many moles of CO2 are produced
Q37: A student weighed 0.550 g of lithium
Q40: The number of grams in 7.00 moles
Q49: How many grams of fluorine are required
Q51: The following equation represents the formation of
Q54: In the reaction 2 C + O2
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents