Diatomic nitrogen is added to the equilibrium system:
N2 (g) + H2 (g) 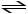
2 NH3(g) + heat
When a new equilibrium is established the concentration of H2 will be ________ the amount at the original equilibrium,and the amount of NH3 will be ________ the amount at the original equilibrium.
A) greater than; greater than
B) greater than; less than
C) less than; greater than
D) less than; less than
E) Both changes will be impossible to determine.
Correct Answer:
Verified
Q61: Match the following.
-A process or reaction that
Q62: For the following reaction,increasing the pressure will
Q63: Match the following.
-A process or reaction that
Q64: If we add a catalyst to the
Q66: Match the following.
-The amount of energy which
Q68: Match the following.
-A process or reaction which
Q69: Match the following.
-A process or reaction which
Q70: Which change to this reaction system would
Q71: The position of the equilibrium for a
Q76: Which of the following conditions characterizes a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents