For the following reaction,increasing the pressure will cause the equilibrium to ________.
2 SO2 (g) + O2 (g) 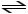
2 SO3 (g) + heat
A) shift to the right,towards products
B) shift to the left,towards reactants
C) remain unchanged,but the reaction mixture will get warmer
D) remain unchanged,but the reaction mixture will get cooler
E) Pressure has no effect on equilibrium.
Correct Answer:
Verified
Q45: Q46: Which factors would increase the rate of Q61: Match the following. Q63: Match the following. Q64: If we add a catalyst to the Q65: Diatomic nitrogen is added to the equilibrium Q66: Match the following. Q71: The position of the equilibrium for a Q76: Which of the following conditions characterizes a Q80: When a reaction system is at equilibrium![]()
-A process or reaction that
-A process or reaction that
-The amount of energy which
A)there
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents