In the reaction shown,what is the mole ratio that would be used to determine the number of moles of oxygen needed to react with 3.2 moles of C4H10?
2 C4H10+ 13O2→ 8 CO2+ 10 H2O
A) 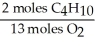
B) 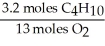
C) 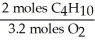
D) 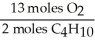
E) 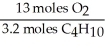
Correct Answer:
Verified
Q24: 50.0 g of Cl2 contains _ mol
Q25: 105 g of MgCl2 contains _ mol
Q26: Determine the number of moles of water
Q27: How many moles of NaHCO3 are present
Q28: Interpret in words the equation shown.
P4O10(s)+ 6
Q30: The combustion of propane gas (C3H8)is used
Q31: How much Ca(NO3)2 should be weighed out
Q32: The combustion of propane gas is used
Q33: The Haber process is used to make
Q34: Consider the reaction N2(g)+ O2(g)→ 2 NO(g).
A)How
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents